Explant Express® Receives FDA 510(k) Clearance for Breast Implant Suction Retrieval Device
PR Newswire
BRECKSVILLE, Ohio, Feb. 12, 2026
FDA-cleared suction retrieval device designed to streamline breast implant removal and reduce mess, handling, and operating time.
BRECKSVILLE, Ohio, Feb. 12, 2026 /PRNewswire/ -- Applied Medical Technology, Inc. (AMT) today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for Explant Express®, a breast implant removal device designed to support efficient, controlled explantation of ruptured silicone breast implants.
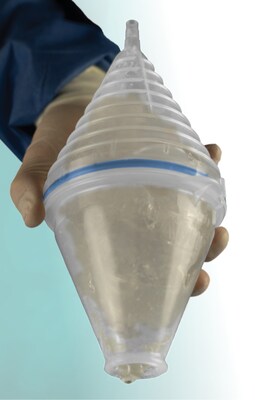
Explant Express® is a breast implant suction retrieval device engineered to help plastic and reconstructive surgeons remove ruptured implants while minimizing contact between silicone gel and the surgical field. By connecting to standard operating room suction, the device simplifies ruptured breast implant explantation without adding capital equipment or requiring advanced training.
"FDA clearance of Explant Express® represents an important milestone in advancing tools that support efficiency, cleanliness, and control during ruptured breast implant removal procedures," said Joseph Harr, Surgical Sales Manager at Applied Medical Technology, Inc. "Traditional explantation techniques can be time-consuming and messy. Explant Express® was designed to reduce operating time and help maintain a clean surgical field - without disrupting existing workflows."
Explant Express® is intended for use during ruptured breast implant explantation procedures and features an intuitive, ergonomic design suitable for a wide range of implant sizes.
Key features of Explant Express® include:
- Rapid removal of ruptured silicone breast implants
- Universal suction compatibility with standard operating room equipment
- Ergonomic, no-slip design suitable for all hand sizes
- Separable chamber allowing post-procedural inspection of retrieved material
Manufactured in the United States, Explant Express® expands Applied Medical Technology's portfolio of FDA-cleared surgical solutions. Commercial availability of Explant Express® will be announced in the coming weeks.
Get the latest product updates and launch information on the FDA-cleared Explant Express® breast implant removal device at https://www.appliedmedical.net/surgical/explant-express/.
About Applied Medical Technology, Inc.
Applied Medical Technology, Inc. (AMT) is a global leader in enteral and surgical devices committed to improving lives through innovation. For over 40 years, AMT has bridged the gap between medical technology and patient needs, collaborating with healthcare professionals and users to develop high-quality, life-enhancing solutions. Our holistic approach prioritizes the well-being of the whole person, not just the device they use.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/explant-express-receives-fda-510k-clearance-for-breast-implant-suction-retrieval-device-302686926.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/explant-express-receives-fda-510k-clearance-for-breast-implant-suction-retrieval-device-302686926.html
SOURCE Applied Medical Technology, Inc.